Abstract
Introduction: Two new systems for the classification of myeloid neoplasms were recently published by the World Health Organization (WHO 5th edition; Khoury et al. 2022) and International Consensus Classification (ICC; Arber et al. 2022). Both systems incorporated the molecular characteristics into myelodysplastic syndrome (MDS) classification. Although some entities are identical between WHO and ICC, several significant differences exist. It is imperative for clinicians, investigators, and pathologists to be mindful of these key differences and their implications in the diagnosis and management of MDS.
Methods: In this single center retrospective study, clinical, pathologic, and genomic data were collected on patients (pts) from the institutional MDS database. All pts treated at Moffitt Cancer Center with available next generation sequencing data at diagnosis were eligible for inclusion. Pts were reclassified by WHO 2022 and ICC 2022 proposed criteria.
Results: Our study included 2231 molecularly annotated pts with MDS. The median duration of follow up was 60.2 months (mo). The MDS with mutated SF3B1 (n=276; 12%), recognized as a unique molecular entity in both WHO and ICC retained its favorable prognostic significance, with the median overall survival (mOS) of 111.6 mo, the best across all subtypes. Similarly, pts with MDS with deletion 5q (n=110; 5%), another category common to both systems experienced a relatively prolonged mOS (75.6 mo).
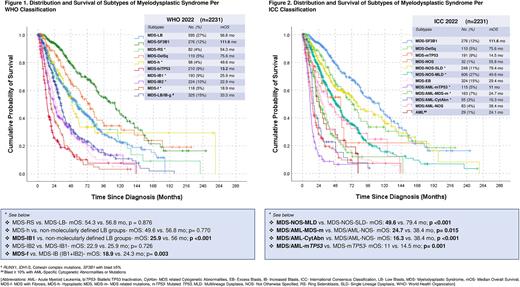
Among other WHO categories (Figure 1), outcome of pts with MDS and ring sideroblasts without SF3B1 mutation (MDS-RS; n=82, 4%) was not significantly different from MDS with low blasts (LB). Newly introduced WHO entity of hypoplastic MDS (n=98; 4%) also had similar mOS compared to non-genetically defined LB group. Pts with MDS with increased blasts-1 (MDS-IB1) had worse survival compared to non-molecularly defined LB groups (mOS- 25.9 vs. 56 mo; p <0.001). However, an increase in blast count ≥10% (MDS-IB2) did not further worsen mOS in our cohort (22.9 vs. 25.9 mo; p= 0.726). The MDS with fibrosis (MDS-f), another unique WHO subtype, had a significantly worse mOS than MDS-IB (IB1+IB2) (18.9 vs. 24.3 mo; p= 0.003).
In the ICC categories (Figure 2), MDS with multilineage dysplasia (MDS-NOS-MLD) had worse mOS (49.6 vs. 79.4 mo; p <0.001) than single lineage dysplasia (MDS-NOS-SLD). Presence of excess blasts ≥ 5% (MDS-EB) shortened survival compared to non-molecularly defined LB groups (mOS- 29.4 vs. 55.7 mo; p <0.001). A blast count ≥ 10% did not confer any significant additional worsening of OS than MDS-EB, in the absence of TP53 mutation. Unique ICC entities of MDS/AML with MDS-related mutations and MDS-related cytogenetic abnormalities had significantly worse mOS than not otherwise specified MDS/AML (24.7 vs. 38.4 mo, p= 0.015; and 16.3 vs. 38.4 mo, p <0.001; respectively).
Categories for TP53-mutated (mTP53) MDS in both classifications had the worst survival of all subtypes. Pts in the WHO mTP53 category requiring bi-allelic TP53 inactivation (n=210; 9%) and two ICC entities based on blast count cut-off of 10%, mTP53-MDS (n=191; 8.5%) and mTP53 MDS/AML (n= 115; 5.1%) had very poor median OS of 13.2, 14.5, and 11 mo, respectively. Within the WHO mTP53 MDS, a blast count ≥ 5% portended a worse mOS than LB population (11.9 vs. 18 mo; p= 0.04), although pts with ≥10% blasts had similar OS to those with 5-9%. Difference in mOS between ICC mTP53 subtypes (<10% vs. ≥ 10%) was statistically significant (p= 0.001), but this was not clinically meaningful (14.5 vs. 11 mo).
Conclusions: We validated the genetic and morphologic WHO and ICC subtypes of MDS. Based on our data, both proposed classifications need to be refined. Molecularly defined entities (SF3B1, deletion 5q, and bi-TP53) are clearly unique. Survival of MDS-RS without SF3B1 was not different from MDS-LB. MDS-MLD had worse OS than MDS-SLD. A blast cut off of 5% correlated with outcome better than 10%, reflecting an accelerated phase disease biology. MDS-f stood out as a distinct morphologic entity with poor OS. The mTP53 status predicted most dismal OS in both systems, and bi-TP53 should be considered a unique entity defined by VAF >50%, two TP53 mutations, single mutation with -17 or complex karyotype. These findings will inform clinicians on the best ways to use WHO and ICC systems in practice and could potentially guide future efforts of reunited MDS classification with a strong impact on clinical trial development.
Disclosures
Tinsley-Vance:Abbvie: Consultancy; Novartis: Consultancy; Jazz: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; CTI: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Sweet:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Dedham Group: Consultancy; Jasper Therapeutics: Consultancy; Astellas: Consultancy; Syntrix Pharmaceuticals: Research Funding; Dava Oncology: Consultancy; Novartis: Consultancy; Agios/Servio: Consultancy; Boxer Capital: Consultancy; Jazz: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. Padron:Blueprint: Honoraria; Kura: Research Funding; Stemline: Honoraria; Incyte: Research Funding; Taiho: Honoraria; BMS: Research Funding; Syntrix Pharmaceuticals: Research Funding. Sallman:Intellia: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Syntrix Pharmaceuticals: Research Funding; Syndax: Membership on an entity's Board of Directors or advisory committees; Nemucore: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Lixte: Patents & Royalties: LB-100. Komrokji:Servier: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Geron: Consultancy; Acceleron Pharma: Consultancy; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.